|
|
Pharmacy and Pharmaceutical Technology section. School of Pharmacy. UMH |
|
English / Spanish |
|
Mission
Group Research and personal contributions
Team
Teaching activities
Collaborations
|
Projects
Ongoing
Projects
Biosim: Network of Excellence. Biosimulation - A New Tool in Drug Development
Past
Projects
Permeability in Caco-2 cultured cells: application to bioavailability predictions (GV99-99-1-12). Influence of Ethanol on drug absorption. Bioavailability predictions on drug development: fluoroquinolones (SAF96-1710). Ongoing Projects
Memtrans Project: “Membrane transporters: in vitro models for the study of their role in drug fate” STREP, funded by EC. The goals of MEMTRANS project are to optimize and pre-validate an in vitro cultured cell models to predict oral absorption and pharmacokinetics of efflux systems substrates (P-gp, MRP2, BCRX for instance), to establish an in vitro cut off for the risk of secretion associated problems and to study the structure-affinity-transport relationships. That would allow 1) a reduction in the number of animal experiments to study efflux processes 2) the in vitro study of drug-drug interactions related with secretion transporters. 3) To obtain useful information for modelling transporter interactions in other normal and transformed tissues (blood brain barrier, lung mucosa, tumours). The objectives of the project will be achieved by using a systems biology approach that involves modeling and simulating the complex dynamic interactions between proteins (transporters), metabolites (i.e substrates) and cells (lipoidal barriers).Computational and mathematical predictive models will be generated from the data based on the system parameters and in the drug characteristics. The experimental data generated in the project will be analyzed from a mechanistical point of view in order to split all the individual steps involved in transport. The mathematical models in combination with the right physiological information could be applied to predict drug transport in a wide range of membranes in the organism (blood brain barrier, liver, kidney, tumors etc) and could be of application for any kind of substrate either drug, toxicants or functional food components. Mentrans FUN-FOOD: : In vitro an in silico models for bioavailability predictions of functional food components. The main goal of this project is to validate the ability of intestinal cell in vitro models to predict oral bioavailability as intestinal permeability is a key factor determining the acces to systemic circulation. In particular we want to study the role of efflux intestinal transporters in the absorption process of functional food components and xenobiotics, because the secretion processes cause the low oral bioavalability of some molecules of nutritional interest and are responsible of clinically relevant food-drug interactions. The basic knowledge generated will be translated in the improvement of the current in vitro methods and in predictive mathematical models (in silico) that will permit the prediction of bioavailability and bioaccesibility from in vitro data. To achieve this goal we propose the following specific objectives: 1. To check the ability of the in vitro cell culture Caco-2 for reproducing the in vivo efflux secretion processes mediated by ABC transporters in order to validate their predictive potential by means of permeability assays of a group of compounds substrate of these efflux transporters. 2. Develop from the current in vitro models a new one that allows the simultaneous estimation of intestinal permeability and first hepatic pass effects in order to predict oral bioavailability. 3. To use the validated in vitro model for estimating the absorption of two functional ingredients, isoflavones and conjugated linoleic acids and the potential absorption changes in the presence of intestinal flora. To achieve this goal: · The metabolism by intestinal flora of isoflavones and linoleic conjugated acids will be characterized. · The changes on intestinal permeability associated to the presence of the flora and due to the induction or inhibition of trasnporters and/or enzymes will be checked. · Other secondary effects associated with the presence of the flora and the diet composition (as the presence of prebiotics) will be studied. 4. The last stage of the project will consist of the correlation analysis and the generation of predictive mathematical models. This includes the relationship among transport parameters, structural features and transporter levels in the cell clone as well as the in vitro-in vivo correlation. Biosim: Network of Excellence. Biosimulation - A New Tool in Drug Development BioSim is a Network of Excellence established by the European Commission under its 6th Framework Programme. BioSim was initiated on December 1, 2004. The main objective of the Network is to demonstrate how the use of modern simulation technique through a deeper and more qualitative understanding of the underlying biological, pathological and pharmacological processes can lead to a more rational drug development process, improved treatment procedures, and a reduction in the needs for animal experiments. With its 26 academic, 10 industrial and 4 regulatory partners, the BioSim Network commands a wide range of biomedical expertise. At the same time, the network involves leading experts in pharmacokinetics, computer simulation, and complex systems theory. The purpose of the network is to develop in silico simulation models of cellular, physiological and pharmacological processes to provide a deeper understanding of the biological processes and help the pharmaceutical industry maintain its competitive power. Marival Bermejo is the coordinator of Activity Area 2 "Regulatory issues and Industry relationships". Her group is coordinating two Workpackages. WP2: The use of modelling and simulation for regulatory decissions" and WP15: "In vitro-in situ and in vivo correlations". Pre- Validation of Permeability studies in Caco-2 cultured cells to predict oral exposure to toxicants and chemicals. The aim of the project is to pre-validate the permeability studies in Caco-2 cells for a group of mdel toxicants and chemical as a method to predict the oral bioavailability of these compounds i.e the exposure risk after oral ingestion. The permeability studies will be developed for a group of toxicants, chemicals and drug models in two laboratories using common SOP in order to evaluate the predictive ability of the model and the interlaboratory variability. Past Projects
Drug Permeability in Caco-2
cultured cells: application to bioavailability predictions. (GV99-99-1-12)
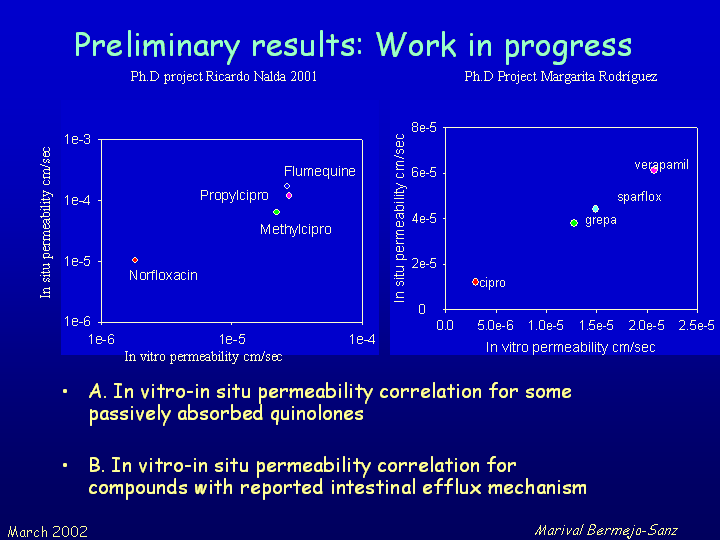
The aim of the project is to determine the permeability coefficients in Caco-2 cells for a serie of quinolone derivatives previously studied in situ and in vivo and validate this in vitro model to predict oral bioavailability of these compounds. The same in vitro model would allow to study the possible influence of P-glycoprotein and other transporters in quinolone absorption.
The first step of the project consist of validating our SOP's through the determination of the permeability values of a number of well studied drugs in other laboratories like betablockers as propranolol, atenolol and metoprolol. The second objective is to determine the permeability values of a group of quinolone derivatives and correlate these values with the absorption rate constants and bioavailability previously determined in rat. We want also further investigate the quantitative importance of efflux mechanism by P-glycoprotein. Our fina goal is to offer our technical support and advice with this methodology to other departments and to the National Industry. The difussion of our results will be achieved by organization of seminars and through the web page of the project (presently in spanish) http://www.uv.es/~mbermejo/proyecto1.htm Some of our preliminary results are shown in the next figures
Validation of a biophysical model
to predict intestinal drug absorption of xenobiotics (PR 82-01563)
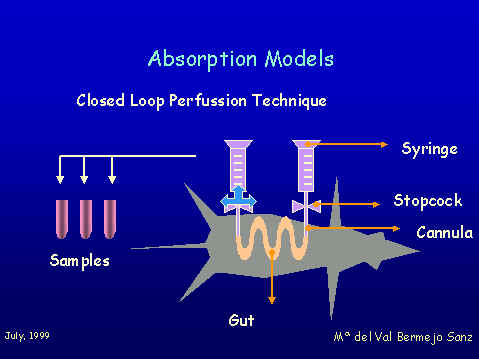
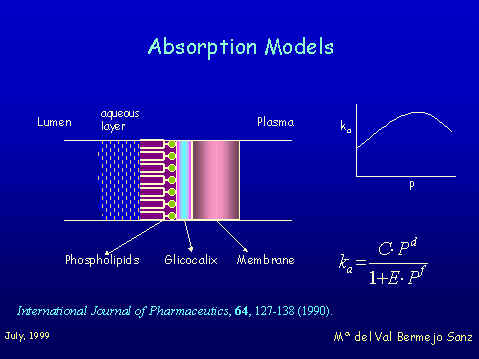
The aim of this project was to asses the absortion model of Professor Pla Defina and Professor Moreno as a tool for describing passive absorption mechanism and to go deeply into the structures of the physiological absorbing barriers. The methodology consist of establishing correlations between absorption rate constants and lipophilicity for a several homologous series of xenobiotics Figure 1 shows the closed loop perfussion technique that was used to obtain intestinal absorption rate constants in rats.
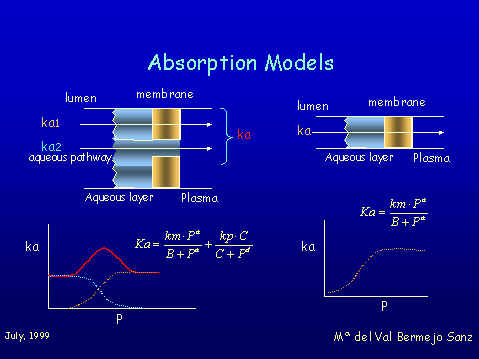
The Model considers passive intestinal absorption as the result of two simultaneous processes: diffusion through aqueous chanels (porous or tight juntions) and diffusion through the lipophilic membrane. The absorption rate constant is the sum of the two parallel processes.
This means that the correlation between ka and P is represented by the sum of two hyperbolic functions, a direct one for the lipidic permeation, and a inverse one for aqueous porous diffusion, with two limiting asymptotic values. The bihyperbolic model of Pla-Delfina can be applied in specialized absorption sites such as small intestine when the two pathways are involved, that is with compounds of molecular weight low enough to use the aqueous pathway. In colon only very small porous for water exchange should exist and in this case we always found hyperbolic correlations. In stomach we found bilineal correlations between Ka and lipophilicity. This kind of relationships are found when heterogeneus barriers systems prevail at the absorption site. This idea seems in agreement with the approaches of Hills and coworkers that described a lipid lining of natural amphiphiles that protects gastric mucosae.
The above structure must act as a heterogeneous system to xenobiotic diffusion (stagnant aqueous layer (hydrophilic), phospholipids (lipophilic), Glycocalyx (Hydrophilic) lipid bilayer (Liphophilic) and aqueous plasma sink), and that produces the bilinear correlation. In summary, the kind of correlation that we have found between ka and P depends on the absorption site. Bilinear in stomach, always hyperbolic in colon and byhyperbolic or hyperbolic in small intestine according to the molecular weight of compounds. Study of the effects of synthetic
and natural surfactants on xenobiotic intestinal absoprtion: (PR-86-0580).
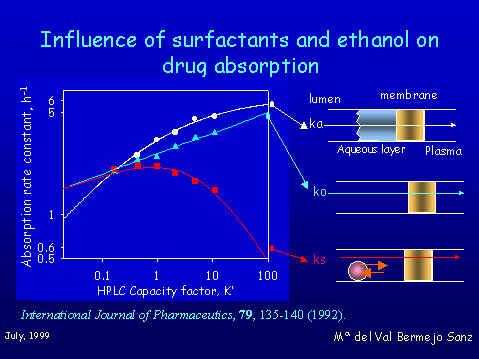
The methodology consist of establishing absorption-partition relationships for homologous series of compounds in presence of two different concentrations of surfactants. Its critical micelle concentration (CMC) and a supramicellar concentration. (SMC) While correlations between ka and P in free solutions are hyperbolic, bihyperbolic or bilinear (depending on the absorption site) correlations in presence of CMC of synthetic surfactants are always potential with a rather low slope.
These results could be interpreted as following: 1. Surfactants seem to nullify the limiting effect on solute diffusion of the aqueous layer 2. Surfactants increase the polarity of the membrane, rendering it more permeable for highly hydrophilic substances. 3. When the surfactant is added at SMC concentration the above effects became almost completely masked by the micellar solubilization of the xenobiotics. Correlations became bilinear as a result of the partitioning process of the solutes between the micellar cores and the aqueous solution When we used a natural surfactant,a bile salt like sodium taurocholate, the ability to eliminate the limiting effect of the aqueous layer is not observed. There is a lesser solubilization potential of taurocholate micelles than polisorbate micelles. Appart from the effects already discussed, other fundamental conclusion of our work is that synthetic and natural surfactants behave in a different way and when this kind of additives are used in a drug formulation its possible influence on drug absorption must be considered. Influence of Ethanol on drug
absorption.
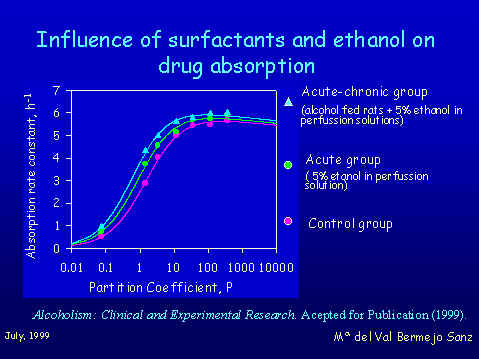
The aim of the study was to investigate the effect on absorption of a series of quinolones of chronic and acute ethanol intake. With the same in situ methodology previously described we stablished correlations between absorption rate constants of the quinolones derivatives and lipophilicity in three groups of animals 1. control group 2. A group with 5% of ethanol in perfussion solutions and 3. chronic alcohol fed rats plus ethanol in the perfussion solutions
The results obtained show that in the two conditions the presence of ethanol produces a fluidizing effect on intestinal membrane. The fluidification leads to an increase of the diffusion of all the compounds, but the more lipophilic compounds do not show any changes in their ka values because they are rate limited by their diffusion in the aqueous boundary layer wich remains unaffected. Bioavailability predictions on
drug development: fluoroquinolones: (SAF96-1710).
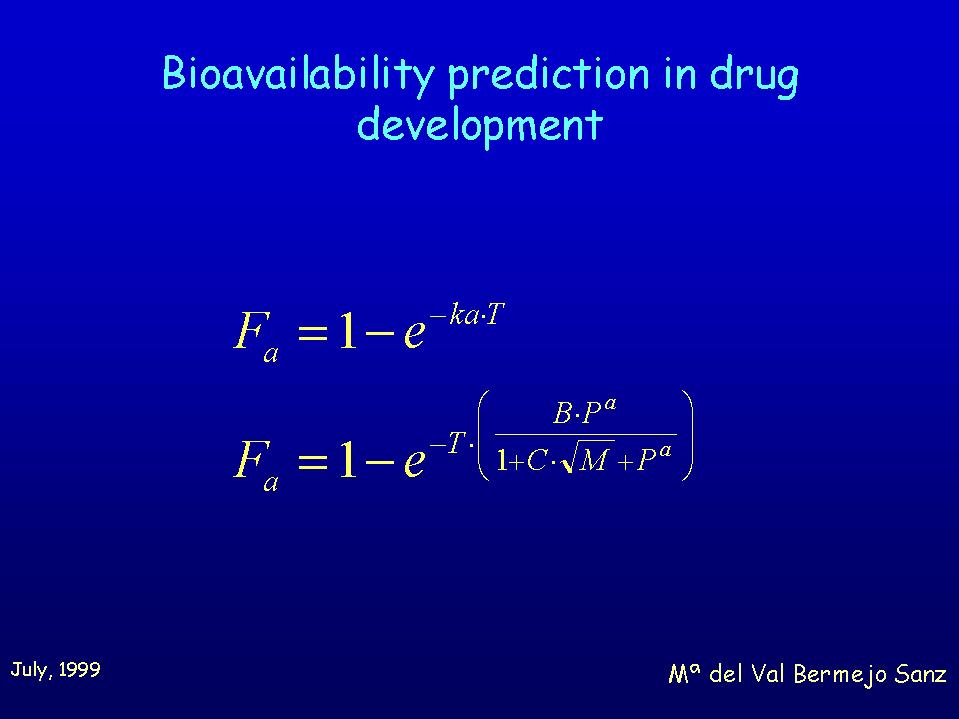
Our aim was to establish a correlation between "in situ" absorption rate constants wich were already determined in the first project and the maximun fraction absorbed in rat. Assuming that there is no first pass effect it could lead to a correlation between ka and bioavailability. We used for the correlation a cumulative equation where F is bioavailability, ka the absorption rate constant and T the time of effective absorption whose maximun value will be intestinal transit time.
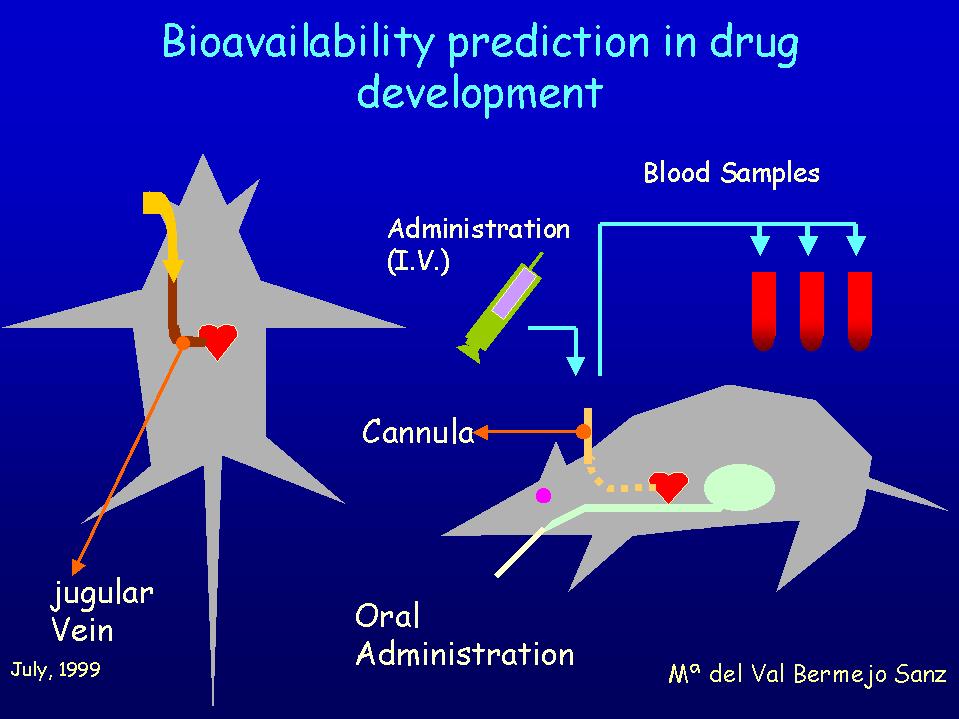
Bioavailability of each compound was determined by means of a permanent jugular vein cannulation technique. This technique allows iv. administration of drug solutions as well as blood sampling. Oral administration was made with the aid of a gastric sonda.
The results obtained at the moment with some homologous quinolones show that the absorption rate constant ka determined in situ in rat is a good predictive parameter of oral bioavailability for the studied compounds.The efflux processes observed in situ and in vitro have not a significant influence in vivo in rats at the oral doses used.
|